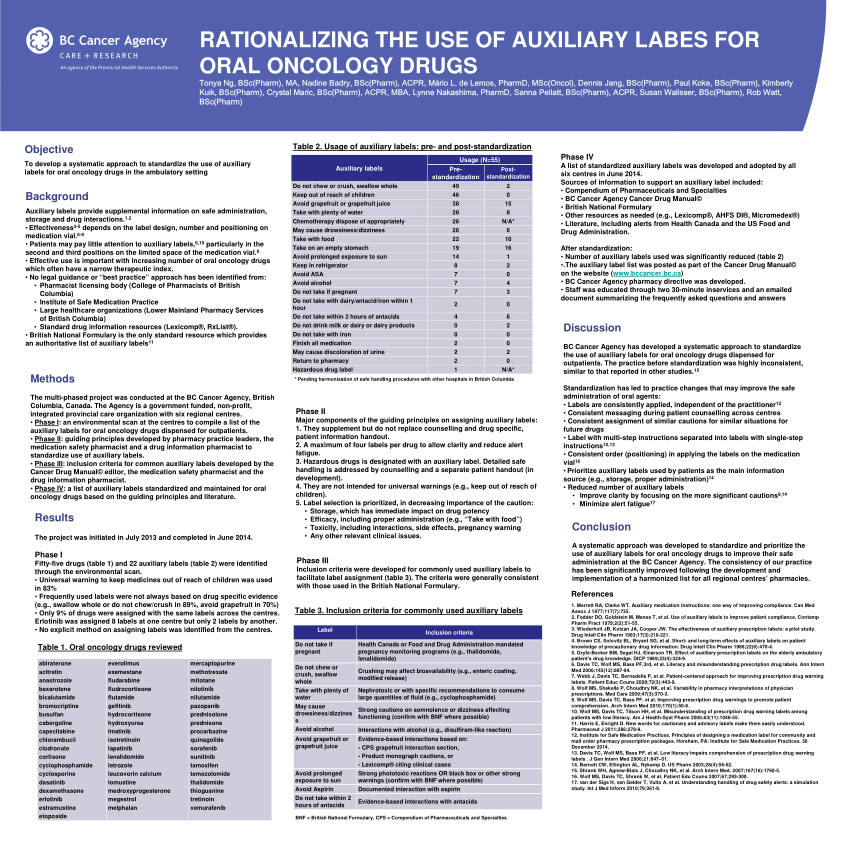
44 cautionary and advisory labels for medicines
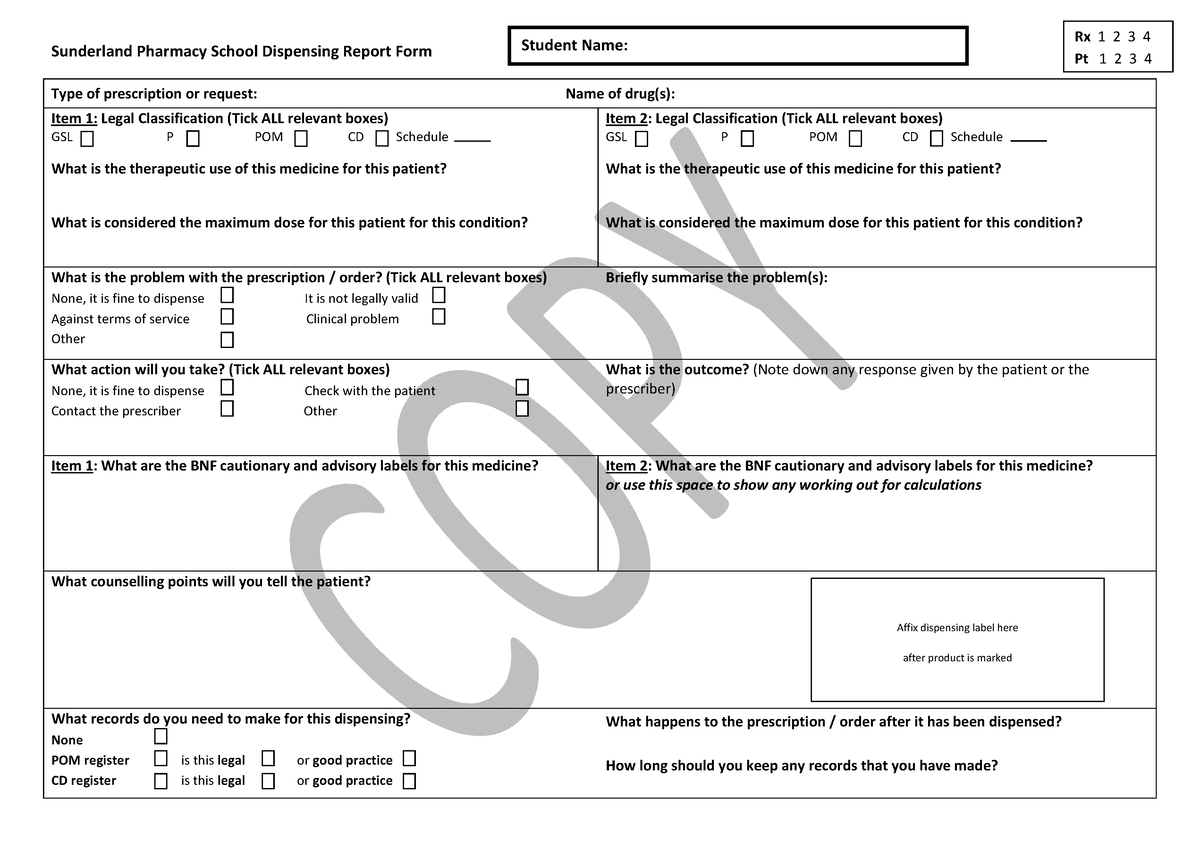
Cautionary Advisory Labels (CAL) - Medi Print Cautionary Advisory Labels (CAL) Categories. Laser Labels. Laser Sheets; Show All Laser Labels. Drug Labels . Burette & Additive Labels ; Drug Identification Labels ; ... This medication may cause drowsiness - CAL Code: CAL-01 Size: 16 x 46mm Quantity: 1,000 per.. Add to Cart. Cautionary_and_advisory_label : definition of Cautionary_and_advisory ... Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels.
Cautionary and advisory labels for dispensed medicines - Blogger Cautionary and advisory labels for dispensed medicines Numbers following the preparation entries in the BNF correspond to the code numbers of the cautionary labels that pharmacist are recommended to add when dispensing. It is also expected that pharmacists will counsel patients when necessary.

Cautionary and advisory labels for medicines
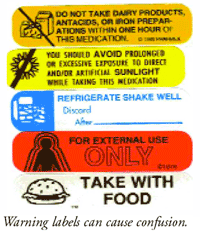
Bagot Press - Pharmacy Consumables & Print Specialists Cautionary and Advisory Label holder: 1 : Cal 1: This medicine may cause DROWSINESS and may ... Cautionary Advisory Labels Flashcards | Quizlet Fluoxetine. o Label 5: Ask your doctor or pharmacist before using any other medicine including over-the-counter medicines or health products. o Label 9: DO NOT STOP TAKING THIS MEDICINE ABRUPTLY unless otherwise stated by your doctor. o Label 12: this medicine may affect mental alertness and/or coordination. 50 Common Warning Labels On Medication Containers According to a consumer report, every year about 500,000 people in American have either misread or misinterpret the medication labels. For some of these individuals, the consequence may not be serious. But for certain cases, the results can be life-threatening. To help minimize this problem, many pharmacies have applied extra warning labels to the container in hope to clarify vital information pertaining to a specific drug.
Cautionary and advisory labels for medicines. Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. PDF cautionary advisory labels - Openbook Howden cautionary advisory labels OBH 18642 CAL's are a valuable tool for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000. Cautionary and advisory labels for medicines - SlideShare Cautionary and advisory labels for medicines 1. CAUTIONARY AND ADVISORY LABELS (CALS) B y Kiran Sharma KIET School of Pharmacy 2. CALs Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. Standard cautionary and advisory labels offer advice but are not exhaustive. Medicinal forms | Clobetasone butyrate | Drugs | BNFC | NICE There can be variation in the licensing of different medicines containing the same drug. Forms available from special-order manufacturers include: cream, ointment. Cream. All products. Show Cautionary and advisory labels. Label 28 . Spread thinly on the affected skin only. Taenwch yn denau ar y croen sydd wedi'i effeithio yn unig. Excipients
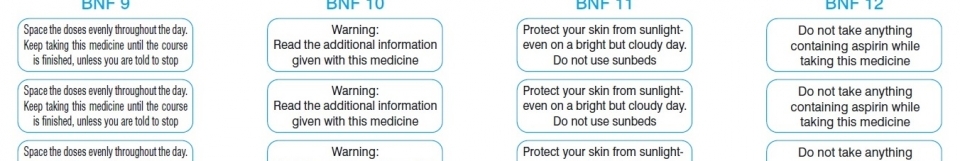
Cautionary and advisory labels | About | BNF | NICE To be used with label 25 on preparations coated to resist gastric acid (e.g. enteric-coated tablets). This is to avoid the possibility of premature dissolution of the coating in the presence of an alkaline pH. Label 5 also applies to drugs such as gabapentin where the absorption is significantly affected by antacids. Pharmacists will be aware (from a knowledge of physiology) that the usual time during which indigestion remedies should be avoided is at least 2 hours before and after the ... PDF Revisions to APF24 Cautionary advisory labels Revisions to Table A2. Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Abacavir Oral solution: 7b (60 days), 12†, 21 Tablet: 12†, 21, A Aciclovir Eye ointment: 7b (28 days) Tablet: D Labels on medicines and poisons - Department of Health Some medicines also require additional label warnings. For example, oral retinoids must have warnings about becoming pregnant. Sedation warnings. Medicines listed in Appendix K of the SUSMP (exernal site) must be labelled with a sedation warning when supplied to patients. Pharmacy cautionary advisory label 1 or label 1A should be used. Cautionary and advisory labels for medicines - Everything2.com Cautionary and advisory labels for medicines. These are the code numbers and their meanings for ...
Required Advisory Statements for Medicine Labels (RASML) Australian labelling requirements for non-prescription medicines (Therapeutic Goods Order No. 92) require some over-the-counter and complementary medicine labels to contain particular warning statements ('advisory statements') about specific risks related to use of the medicines. These advisory statements are set out in the TGA document 'Required Advisory Statements for Medicine Labels' (RASML). Cautionary And Advisory Labels - Medico Pak Cautionary And Advisory Labels These labels are available in eye-catching fluro orange with different statements or instructions. We recommend using these labels to assist care facilities to maximise the safety by affixing appropriate labels to the pack where necessary. Labels measure 40mm x 20mm. Medicine labels - what do the warning statements mean? | Health ... Taking some medicines at the same time as indigestion remedies (antacids), iron or calcium may prevent your body absorbing the medicine, and it may not work as well as it should. The warning about calcium includes vitamins and medicines that contain calcium and calcium-containing food such as milk and cheese. Guidance for cautionary and advisory labels | About | BNF | NICE Recommended label wordings. For BNF 61 (March 2011), a revised set of cautionary and advisory labels were introduced. All of the existing labels were user-tested, and the revised wording selected reflects terminology that is better understood by patients. Wordings which can be given as separate warnings are labels 1-19, 29-30, and 32.
Cautionary and Advisory Label | Cautionary Advisory Label | Technology ... Cautionary And Advisory Label. Cautionary and advisory labels (Cals) are sometimes added (with the dispensing label) to a medicine dispensed by the pharmacist to the patient. A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory ...
Cautionary and Advisory Label | Cautionary Advisory Label Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counselling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know.
Label Statements Database - Medsafe This database lists the warning and advisory statements that are required on medicine and related product labels under regulations 13 (1) (i) and 14 (1) (f) of the Medicines Regulations 1984. Words of a similar meaning to the statements in the database may be used and individual statements may be combined provided the intent is not changed.
Cautionary_and_advisory_label - chemeurope.com A dispensing label is always added to a medicine to show the essential details (the name of the medicine, the dose, and the frequency of administration), and in some cases the pharmacist will add cautionary and advisory labels. Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counselling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the ...
Cautionary and advisory labels for medicines (thing) These are the code numbers and their meanings for the cautionary labels used by pharmacists when dispensing medicines in the UK. Extra counselling may be given with relation to age, experience, background and understanding of the patient.. Warning. May cause drowsiness. To be used on preparations for children containing antihistamines or where label 2 would be inappropriate.
Safer dispensing labels for prescription medicines Australia has a national 'standard icon system' for cautionary advisory labels. See the Australian Pharmaceutical Formulary and Handbook for ... -centred labels. 12-14 One trial randomised 845 participants to receive a patient-centred label or standard dispensing label for their medicine. 12 The study assessed whether participants could ...
StirlingFildes | HealthCare The StirlingFildes Cautionary Advisory Label (CALs) system produced by the PSA is a valuable labelling system designed for helping pharmacists to fulfil their legal and professional ... Warning Label No.9 - Medicine Abruptly Code: WARN 9: 1,000 per box 40 x 11mm: $10.45 (excl GST) Warning Label No.10a - One Aspirin or Tablet Code: WARN 10A:
StirlingFildes | Printing, Packaging and Consumables Warning Label No. 1A Code: WARN 1A: 1,000 per box 46 x 16mm: Warning Label No. 2 Code: WARN 2: 1,000 per box 40 x 11mm: Warning Label No. 3A Code: WARN 3A: 1,000 per box 40 x 11mm: Warning Label No. 3B Code: WARN 3B: 1,000 per box 40 x 11mm: Warning Label No. 4A Code: WARN 4A: 1,000 per box 40 x 11mm: Warning Label No. 4B Code: WARN 4B: 1,000 per box 40 x 11mm
Cautionary and Advisory Label - Label Wording and Warnings The correct storage of a medicine. Dissolution of the medicine in water before taking it. Limits to the number of tablets that should be taken in a given time. Recommended label wording can offer warnings about: Effects of the medicine on driving or work (e.g. through drowsiness). Foods or medicines that should be avoided.
Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do?
50 Common Warning Labels On Medication Containers According to a consumer report, every year about 500,000 people in American have either misread or misinterpret the medication labels. For some of these individuals, the consequence may not be serious. But for certain cases, the results can be life-threatening. To help minimize this problem, many pharmacies have applied extra warning labels to the container in hope to clarify vital information pertaining to a specific drug.
Cautionary Advisory Labels Flashcards | Quizlet Fluoxetine. o Label 5: Ask your doctor or pharmacist before using any other medicine including over-the-counter medicines or health products. o Label 9: DO NOT STOP TAKING THIS MEDICINE ABRUPTLY unless otherwise stated by your doctor. o Label 12: this medicine may affect mental alertness and/or coordination.
Bagot Press - Pharmacy Consumables & Print Specialists Cautionary and Advisory Label holder: 1 : Cal 1: This medicine may cause DROWSINESS and may ...


































Post a Comment for "44 cautionary and advisory labels for medicines"