44 fda approved health claims on food labels
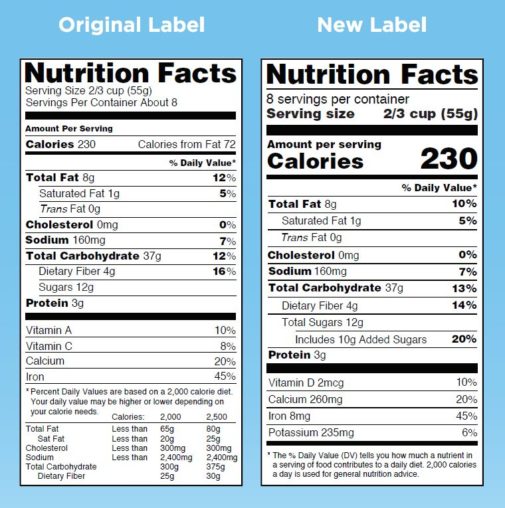
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and; structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and Changes to the Nutrition Facts Label | FDA - U.S. Food and ... Mar 07, 2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
Food Labeling: Health Claims; Dietary Guidance - Federal Register 1. The NLEA authorized health claims in food labeling by amending the Federal Food, Drug, and Cosmetic Act (the act) to add section 403(r) to the act (21 U.S.C. 343(r)). This section specifies, in part, that a food is misbranded if it bears a claim that expressly or by implication characterizes the relationship of a nutrient to a disease or ...

Fda approved health claims on food labels
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Is It Really 'FDA Approved'? - U.S. Food and Drug Administration May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Is Pfizer’s FDA-approved COMIRNATY Vaccine Available in the US? Aug 08, 2022 · On August 23, 2021, the US Food and Drug Administration (FDA) approved Pfizer’s biological licensing application (BLA) for its covid-19 vaccine named COMIRNATY for people aged 16yrs and older. At the time, vaccine hesitancy was persistent and the acting FDA Commissioner Janet Woodcock said that granting full approval to the vaccine might “instill additional …
Fda approved health claims on food labels. Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990... Understanding Health Claims on Food Labels - Food Smart Colorado Health Claim Definitions In addition to the nutrition facts and ingredient information found on packaged foods, some foods may also be labeled with health-related claims. There are three main categories of claims defined by and regulated by the Food and Drug Administration (FDA): Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Understanding Date Labels — FDA Reader May 24, 2019 · The American food consumer is misreading date-labels and mistakenly throwing out $32 billion dollars worth of food because of it. The FDA is trying to minimize food waste and so they are providing specific guidance to manufacturers about how to write date labels and guidance to consumers about how to interpret them properly. Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim" Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Pet Food Labels - General | FDA The term "natural" is often used on pet food labels. AAFCO has developed a feed term definition for what types of ingredients can be considered “natural” and “Guidelines for Natural Claims ... Label Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... CFSAN Constituent Updates | FDA - U.S. Food and Drug ... Aug 11, 2022 · News and announcements related to food, cosmetics, and dietary supplements from the Center for Food Safety and Applied Nutrition at FDA Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Understanding Food Labels | The Nutrition Source | Harvard T.H. The FDA has approved 12 health claims on food labels such as the relationship between calcium and osteoporosis; sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and folic acid and neural tube defects. However, just because a food contains a specific nutrient that is associated with a decreased risk of disease ...
Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ...
Small Entity Compliance Guide on Structure/Function Claims | FDA On January 6, 2000, the Food and Drug Administration (FDA) published a final rule in the Federal Register defining the types of statements that may be used on the label and in the labeling of ...
Questions and Answers on Health Claims in Food Labeling | FDA 4. Has the FDA ever revoked an authorized health claim? The FDA has authorized 12 health claims since 1990. On October 31, 2017, the agency issued a proposed rule to revoke the regulation that authorizes the use of a health claim about the relationship between soy protein and the reduced risk of coronary heart disease.
FDA updates on hand sanitizers consumers should not use Jul 27, 2022 · FDA encourages health ce professionals, consumers and patients to report adverse events or quality problems experienced with the use of hand sanitizers to FDA’s MedWatch Adverse Event Reporting ...
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA A health claim requires FDA evaluation and authorization prior to its use. A structure/function claim describes the role of a substance intended to maintain the structure or function of the body. Structure/function claims do not require preapproval by FDA. 21 CFR 101.14 (a) (1) and (c), and 21 CFR 101.93 (f)
FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA).
Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, …
Health Claims on Food Labels The health claims must be balanced and based on current, reliable scientific studies. And the claims must be approved by the U.S. Food and Drug Administration (FDA). Health claims may be statements like: "This food is a good source of calcium. Adequate intake of calcium may reduce the risk of osteoporosis."
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim".
Is Pfizer’s FDA-approved COMIRNATY Vaccine Available in the US? Aug 08, 2022 · On August 23, 2021, the US Food and Drug Administration (FDA) approved Pfizer’s biological licensing application (BLA) for its covid-19 vaccine named COMIRNATY for people aged 16yrs and older. At the time, vaccine hesitancy was persistent and the acting FDA Commissioner Janet Woodcock said that granting full approval to the vaccine might “instill additional …
Is It Really 'FDA Approved'? - U.S. Food and Drug Administration May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ...











Post a Comment for "44 fda approved health claims on food labels"